Learning styles and chemistry
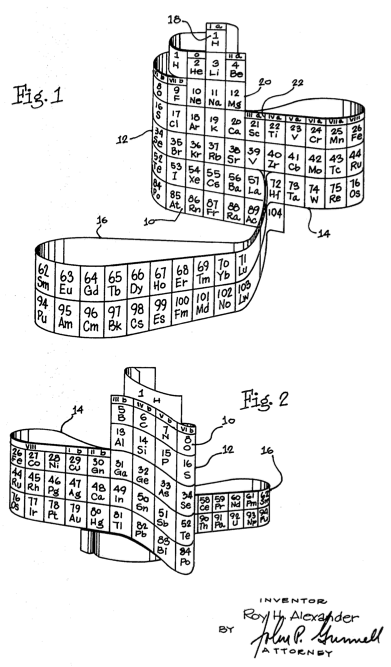
Roy Alexander, creator of the 3-D Periodic Table, recently commented on my blog since I had posted previously about his unique construction. Below is from his “The Argument for 3-D Periodic Table” (emphasis original):
The theory of multiple intelligences has grabbed the attention of many educators around the country, and hundreds of schools are currently using its philosophy to apply to the various student learning styles.
Students generally fall into more than one of Gardner’s multiple intelligences. Those other than word-, number-, and reasoning-smart may deliberately avoid or fail at the ordinary academics. Teachers, most of them of the above intelligences, may have difficulty perceiving the needs of the others. It is important to address all the students, in Chemistry as in other school subjects.
All students have some of all the intelligences, most leaning more toward one or several. A 3-D model of the periodic table touches on some of the intelligences often ignored, as well as addressing students capable of critical thinking. The periodic table is introduced at the beginning of chemistry, and so is of great importance.
The construction of the 3-D model, an interactive and kinesthetic activity, has the student physically handle specific segments of the table, and deliberately place them in their correct locations relative to all the other parts, helping them to learn the names of the segments and see how obvious and logical the periods are.
The naturalist in students can identify the dimensionality of the periodic table with all other parts of nature - which are all also three dimensional. This association removes the table from the purely abstract to represent, as it informationally does, all of reality.
In a classroom session, the common experience of all to make their own periodic table (and to have it for themselves - perhaps to show to parents, siblings, etc. …and have to then explain something about it) provides, initially, an interpersonal experience with classmates, and then a sense (and reality) of ownership. The flat tables will always be there for quick and easy reference, but the foundation for that table, will be solid - literally.
The pictured 3-D models used in the introduction to chemistry, promote quick and easy familiarity with the periodic table for the new student.
Students characterized by the first of Gardner’s three intelligences; “ Linguistic (“word smart”), Logical-mathematical (“number/reasoning smart”), and Spatial intelligence (“picture smart”), can have the speed and clarity of their understanding of the flat table in universal use enhanced by enlisting other parts of their own intelligences.